Molecular Switches |
|
|
|
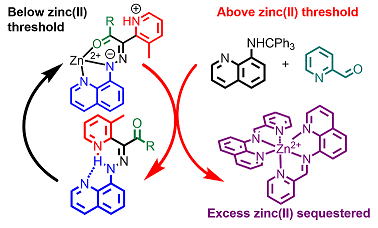
We have been expanding the tool-box available for practitioners in the field of molecular switches and machines by developing chemically and/or photochemically activated hydrazone-based rotary switches. As part of this effort, we developed coordination-coupled deprotonation (CCD) as a mechanism for initiating multi-step switching cascades that can be used in controlling catalysis, signaling and signal amplification processes, among other possibilities. The most complicated cascade developed so far is the earliest instance of a synthetic negative feedback loop, which is a promising candidate for the future design of controllable out-of-equilibrium systems. The ultimate goal of this systems chemistry approach to molecular switches, is the design of reaction cascades that can ultimately lead to complexity.
|
|
Most recently we developed a new family of bistable photochromic hydrazones that exhibit emission toggling and solid-state switching. So far, we used these systems in kinetically trapping the i) self-assembly of liquid crystals, resulting in an optical window application, and ii) actuation of a liquid-crystalline elastomer, resulting in shape-persistent actuators that deform in stages. These results show how the development of new molecular switch architectures brings about functionality that hitherto was not possible.
|
|
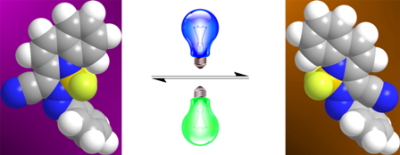
An offshoot of the hydrazone-based switch project is the development of a azo-BF2 complexes that can be switched between the trans and cis configurations using solely visible and/or near infrared (NIR) light. These switches are promising candidates for use in photopharmacology, as they do not rely on deleterious UV light in their activation. Moreover, the deeper penetration of visible and/or NIR light into substrates such as polymers make these switches ideal for polymer actuation.
|